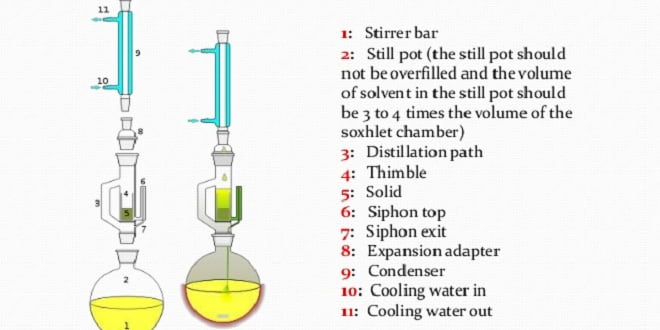
Extraction
- Meyve ve Sebzeler

Turunçgil Kabuklarının Biyoaktif Bileşenleri ve Antioksidan Aktivitelerinin Belirlenmesi ( Melih GÜZEL )
Turunçgil Kabuklarının Biyoaktif Bileşenleri ve Antioksidan Aktivitelerinin Belirlenmesi Melih GÜZEL , Özlem AKPINAR Öz Meyve ve sebzelerde bulunan biyoaktif bileşenlere karşı ilgi,…
- Genel Kimya

Acid / Base Extraction and Separation of Acidic and Neutral Substances
Experiment 3: Acid/base Extraction and Separation of Acidic and Neutral Substances Introduction You will be given a mixture that contains three substances…
- Yağ Teknolojisi

Extraction And Determination Of Crude Fat From Plant And Animal Tissues ( Ms. Nadia Amara )
Extraction And Determination Of Crude Fat From Plant And Animal Tissues Ms. Nadia Amara Introduction of Crude Fat : Determination of Crude…
- Yağ Teknolojisi

Extraction & Determination of Crude Fat From Plant or Animal Tissues ( ABDALQADER A. ABBAS )
INTRODUCTION The term “lipid” refers to a group of compounds that are soluble in water, but show variable solubility in a number…
- Organik Kimya

Laboratory > Liquid – Liquid Extraction
INTRODUCTION When a solution of a solute in one solvent is shaken with a second solvent, which is immiscible with the first,…
- Gıda Uygulamaları Laboratuarı

Solid Liquid Extraction ( Ali OK )
Firstly, some amount of dried hazelnut sample was taken and put into the mixer to grind and to reduce the particle size.…
- Gıda Uygulamaları Laboratuarı

Solid-Liquid Extraction for Pharmaceutics, Food Processing and Pulp Industry ( W.M. Zadorsky )
1. Introduction Vegetable oils, sugar, instant coffee, medicines from medicinal plants, etc. are made by processing solid starting material using extraction with…